The end result is adulterated supplements on the marketplace.
I wish people were not so naive as to believe a PDF document, said Oskar Thorvaldsson with VeriGMP. Brand owners and people working for supplement brands often ask manufacturers if they can email them a PDF of their GMP certification. This is a horrible idea!
Never trust a GMP certification unless you get it by clicking a link to the certifying body. Not only can PDFs be easily manipulated, in the case of NSF they don’t tell the entire story. It’s the same thing with Certificate of Analysis!
The fact of the matter is that for years the certifications that NSF issues are exactly the same for three classes of facilities, manufacturers, distributors, and warehouse facilities.
“That’s right they are absolutely identical”, said Oskar Thorvaldsson, CEO of VeriGMP, he maintains that, “Because it’s about 100 times more expensive to obtain a GMP certification for a manufacturing facility than it is for a warehouse or distribution facility, many factories or what you call contract manufacturing organizations (CMO) out there mislead their customers by talking about GMP certification for a manufacturing facility next to a link to an image of their GMP certification from NSF that they received for registering with NSF as a warehouse facility. This is actually very common!”
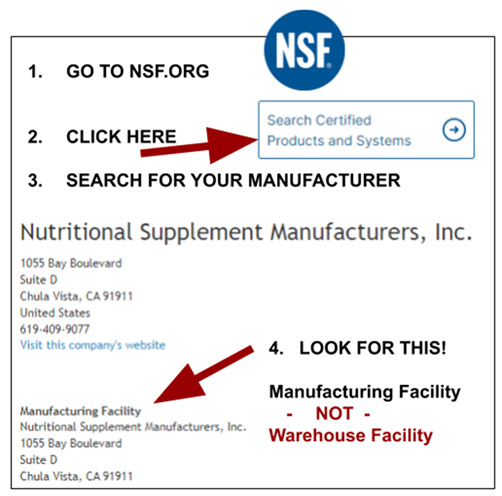
Directions to Label Owners on How Not Get Scammed
Please look at the image. Here are the directions on how to avoid being scammed by a CMO. Here are the questions you need to ask:
- What is the address I pick the products up at? Is this the same address its manufactured at?
- Is your facility GMP certified by NSF?
- Under what name are they certified?
- Go to www.NSF.org
- Locate “Search Certified Products and Systems” in upper right corner
- Type in the name of the facility in the search bar
- If they are registered then they will show up
- They list the address of the facility twice (the same address)
- Compare that address to the address the CMO gave you
- Look above the second time NSF list the address
- It is there that it’s stated if this is a GMP certification for: a) Manufacturing Facility, b) Warehouse Facility, c) Distribution Facility
- It’s a good idea to google map the facility so you can gauge the size
Oskar Thorvaldsson was told by a NSF Senior Account Manager, Health Sciences Certification, Jen Gibbons that: “Further confusion will (also) be resolved with the transition to the NSF/ANSI 455 program as all product technologies and types will be listed on both the NSF public listing page as well as each facility’s certificate.”
What is needed in the supplement industry above all else is a system that enables consumers to trust brands again. This is now completely absent. VeriGMP provides an excellent solution!
Oskar Thorvaldsson, CEO of VeriGMP Tweet




